lewis structure for na|Lewis Structure Finder : Bacolod Learn how to write Lewis symbols and structures for atoms using dots to represent valence electrons. Find out the number of valence electrons for each element from the periodic . Ninoy Aquino International Airport is Manila’s primary airport situated only 7 km from the city. If you are booking flights from Dubai to Manila (DXBA to MNL) you can make the journey with Cebu Pacific, Emirates and Philippine Airlines.Be advised that all flights to Manila from Dubai depart from Dubai International Airport, the country’s primary airport .
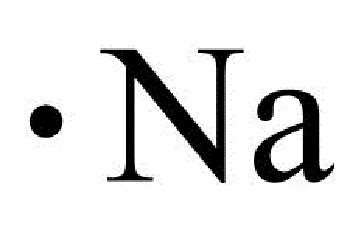
lewis structure for na,A step-by-step explanation of how to draw the Lewis dot structure for Na (Sodium). I show you where Sodium is on the periodic table and how to determine how . A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom .lewis structure for naGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Lewis Structure Finder Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Learn how to write Lewis symbols and structures for atoms using dots to represent valence electrons. Find out the number of valence electrons for each element from the periodic . A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. .
The Lewis dot diagram for sodium consists of the symbol “Na” surrounded by a single dot, representing the valence electron. The Lewis dot diagram for sodium can be further .
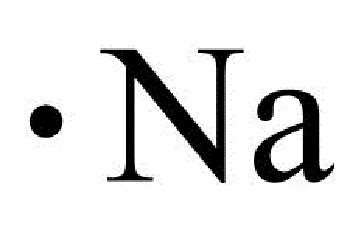
In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. Lewis Symbols We use .
Learning Objectives. By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions. Draw Lewis structures depicting the bonding in simple molecules. Thus far in this chapter, we . For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. . The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion.Lewis Structure Finder. Added Jun 9, 2014 by Tester in Chemistry. This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds. This suggests the best Lewis structure has three B–F single bonds and an electron deficient boron.The Lewis structure of XeF2 XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe Xe atom: XeF6 XeF 6: We place three lone pairs of electrons around each F F atom, accounting for 36 electrons. Two electrons remain, and this lone pair is placed on the Xe Xe atom: Check Your Learning. Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .
A step-by-step explanation of how to draw the NaI Lewis Dot Structure.For NaI we have an ionic compound and we need to take that into account when we draw th.The Lewis Structure for the Salt NaCl depicts two ions with complete octets in their outer shells of electrons. As a result, there is a positive charge on sodium (due to one lost electron) and a negative charge on chlorine (due to one electron gained). . (Na) is the metal that will give away the one electron and forms Na + ion, .
Lewis structure: diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion. Lewis symbol: symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion. lone pair: two (a pair of) valence electrons that are not used to form a covalent bond. The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by .
Question #31400. Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any . Learn how to draw the Lewis Dot Structure for Na3N, an ionic compound, with this easy tutorial. Watch the video and follow the steps.lewis structure for na Lewis Structure Finder A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the .The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what . NaCl lewis structure has overall eight valence electrons available on it. Na has 1 valence electron and Cl atom has 7 valence electrons. Both Na and Cl belong to 1 st A and 7 th A group in periodic table. Thus, Na and Cl atoms shows valence shell electronic configuration as 3s 1 (Na) and 3s 2, 3p 5 (Cl).. Calculation for NaCl lewis structure .
The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a license and was authored, remixed, and/or curated by LibreTexts. The tendency to form species that have eight electrons in the valence shell is called the octet .For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. . The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion.
A step-by-step explanation of how to draw the NaH Lewis Dot Structure.For NaH we have an ionic compound and we need to take that into account when we draw th.For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. . The Lewis Structure of this reaction is (here we will consider one chlorine atom, rather than Cl 2) is: The arrow indicates the transfer of the electron from sodium to chlorine to form the Na + metal ion and the Cl-chloride ion.
lewis structure for na|Lewis Structure Finder
PH0 · The Sodium Lewis Dot Diagram: Understanding the Bonding Patterns o
PH1 · The Sodium Lewis Dot Diagram: Understanding the Bonding
PH2 · Lewis Structure Finder
PH3 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols for At
PH4 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols
PH5 · Lewis Dot Structure for Sodium (Na)
PH6 · How to Draw the Lewis Dot Structure for Na+ (Sodium ion)
PH7 · 9.3: Drawing Lewis Structures
PH8 · 9.2: Lewis Electron Dot Diagrams
PH9 · 7.3 Lewis Symbols and Structures
PH10 · 5.3: Lewis Diagrams
PH11 · 4.4 Lewis Symbols and Structures – General